In the "1KFG: Deep Sequencing of Ecologically-relevant Dikarya" project (CSP1974), we aim to sequence additional sampling of genomic diversity within keystone lineages of plant-interacting fungi and saprophytic fungi that are of special ecological importance for understanding terrestrial ecosystems. In addition, comparative genome analysis with saprotrophic, mycorrhizal and pathogenic fungi will provide new insights into the specific and conserved adaptations associated with each fungal lifestyle.
Within the framework of CSP1974, we are sequencing phylogenetically and morphologically diverse species of Morchellaceae. These fungi include economically important edible morels (Morchella), putatively toxic false-morels (Verpa), and the edible hypogeous truffle genera Leucangium and Kalapuya (1–3). The ecology of these taxa is still poorly understood. Some Morchella species are suspected to be mycorrhizal symbionts (4), others grow as endophytes within plant roots (5,6), some farm bacteria (7), but the majority of species (including the cultivated species of morels) are considered to be general saprotrophs (8,9). The Morchellaceae lineage is hypothesized to have originated and radiated in the Northern Hemisphere, later dispersing into Southern hemisphere regions (10). Genomic data generated by this project will be used to better identify genomic features underlying the distinct ecology, diversity, and morphology of Morchellaceae fungi.
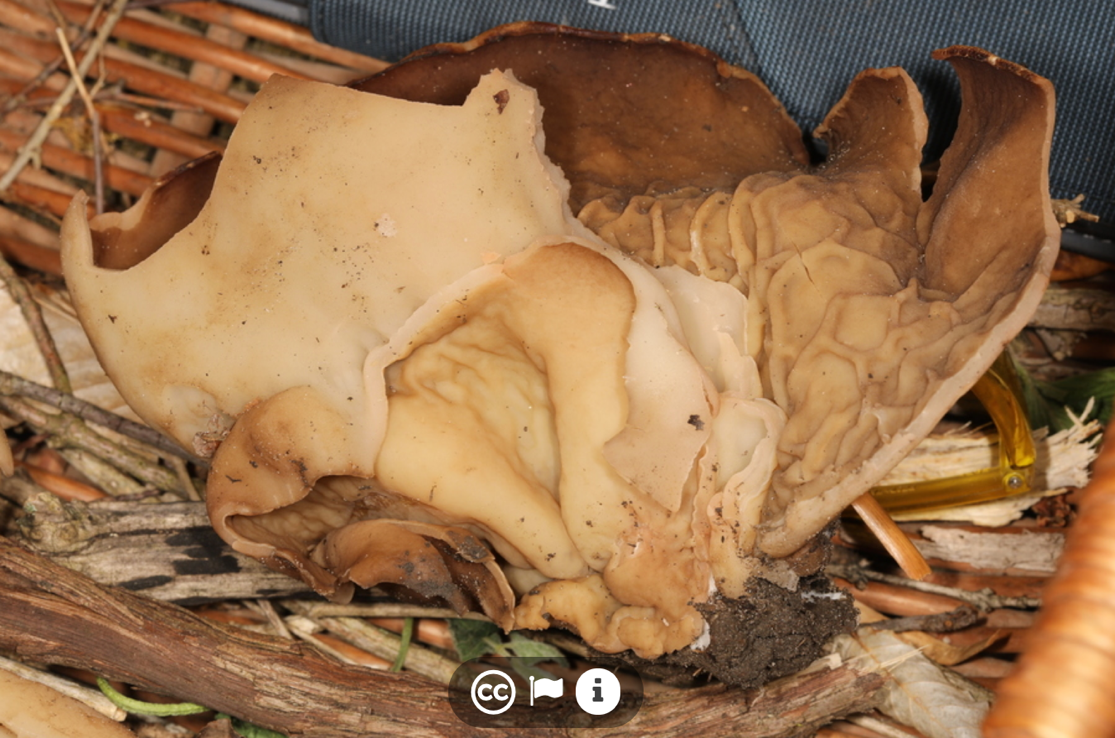
Here we present the genome of Disciotis venosa NRRL 24433 (Pers. ex Pers.), which is commonly known as the 'cup morel.' D. venosa was first described from Klagenfurt, Austria as Peziza venosa (11) and was later transferred to Disciotis (12), within Morchellaceae. D. venosa can be found growing on the ground among mossy or needle-covered soil among conifers, and is more common along banks or slopes, rather than flat ground (13). Young fruiting bodies are usually shallow cup-shaped with a very short stem. Ascocarps are split, with the extreme margin slightly elevated and will flatten down to lie on the soil when they are 7-8 cm in diameter (13). At maturity, the hymenium is dark brown, and may become folded into vein-like markings (Fig 1). The exterior surface has a whitish pubescens, with small brown scales, and ascocarps may be up to 20 cm (8 in) in diameter (14). Mature asci contain 8-spores that are smooth, inamyloid, and lack oil drops.
Researchers who wish to publish analyses using data from unpublished CSP genomes are respectfully required to contact the PI and JGI to avoid potential conflicts on data use and coordinate other publications with the CSP master paper(s).
References
- O’Donnell, K., Cigelnik, E., Weber, N. S. & Trappe, J. M. Phylogenetic Relationships among Ascomycetous Truffles and the True and False Morels Inferred from 18S and 28S Ribosomal DNA Sequence Analysis. Mycologia 89, 48–65 (1997).
- Gecan, J. S. & Cichowicz, S. M. Toxic Mushroom Contamination of Wild Mushrooms in Commercial Distribution. J. Food Prot. 56, 730–734 (1993).
- Trappe, M. J., Trappe, J. & Bonito, G. Kalapuya brunnea gen. & sp. nov. and its relationship to the other sequestrate genera in Morchellaceae. Mycologia 102, 1058–1065 (2010).
- Buscot, F. Mycorrhizal succession and morel biology. Mycorrhizas in ecosystems 220–224 (1992).
- Masaphy, S., Zabari, L., Goldberg, D. & Jander-Shagug, G. The complexity of Morchella systematics: a case of the yellow morel from Israel. Fungi 3, 14–18 (2010).
- Baynes, M., Newcombe, G., Dixon, L., Castlebury, L. & O’Donnell, K. A novel plant–fungal mutualism associated with fire. Fungal Biol. 116, 133–144 (2012).
- Pion, M., Spangenberg, J. E., Simon, A., Bindschedler, S., Flury, C., Chatelain, A., Bshary, R., Job, D. & Junier, P. Bacterial farming by the fungus Morchella crassipes. Proc. Biol. Sci. 280, 20132242 (2013).
- Benucci, G. M. N., Longley, R., Zhang, P., Zhao, Q., Bonito, G. & Yu, F. Microbial communities associated with the black morel Morchella sextelata cultivated in greenhouses. PeerJ 7, e7744 (2019).
- Hobbie, E. A., Rice, S. F., Weber, N. S. & Smith, J. E. Isotopic evidence indicates saprotrophy in post-fire Morchella in Oregon and Alaska. Mycologia 108, 638–645 (2016).
- O’Donnell, K., Rooney, A. P., Mills, G. L., Kuo, M., Weber, N. S. & Rehner, S. A. Phylogeny and historical biogeography of true morels (Morchella) reveals an early Cretaceous origin and high continental endemism and provincialism in the Holarctic. Fungal Genet. Biol. 48, 252–265 (2011).
- Persoon CH. Synopsis methodica fungorum (in Latin) (1801). Göttingen, Sweden. p. 638.
- Rapport sur les excursions faites par la Société Mycologique de France pendant la session de 1893. Bulletin de la Société Mycologique de France (in French). 9 (2): 111. 1893.
- Brown RP. "Observations of Sarcoscypha coccinea and Disciotis venosa in North Wales during 1978–1979". Bulletin of the British Mycological Society. 14 (2): 130–135 (1980).
- Seaver FJ. Photographs and descriptions of cup-fungi V. Discina venosa. Mycologia. 9 (2): 53–54 (1917).
